News
Japan Approves Alzheimer’s Treatment Drug ‘Leqembi’ Amidst Aging Population Crisis
(CTN NEWS) – In a significant stride towards tackling Alzheimer’s disease, Japan’s Ministry of Health has given its seal of approval to Leqembi, a groundbreaking medication jointly developed by Japanese pharmaceutical giant Eisai Co. and the U.S. biotechnology firm Biogen Inc.
This momentous decision marks a pivotal moment not only for Alzheimer’s treatment but also for Japan’s rapidly aging population.
With this approval, Japan takes its first step into a new era of Alzheimer’s care, offering hope to countless individuals and their families affected by this debilitating condition.
Leqembi’s Significance
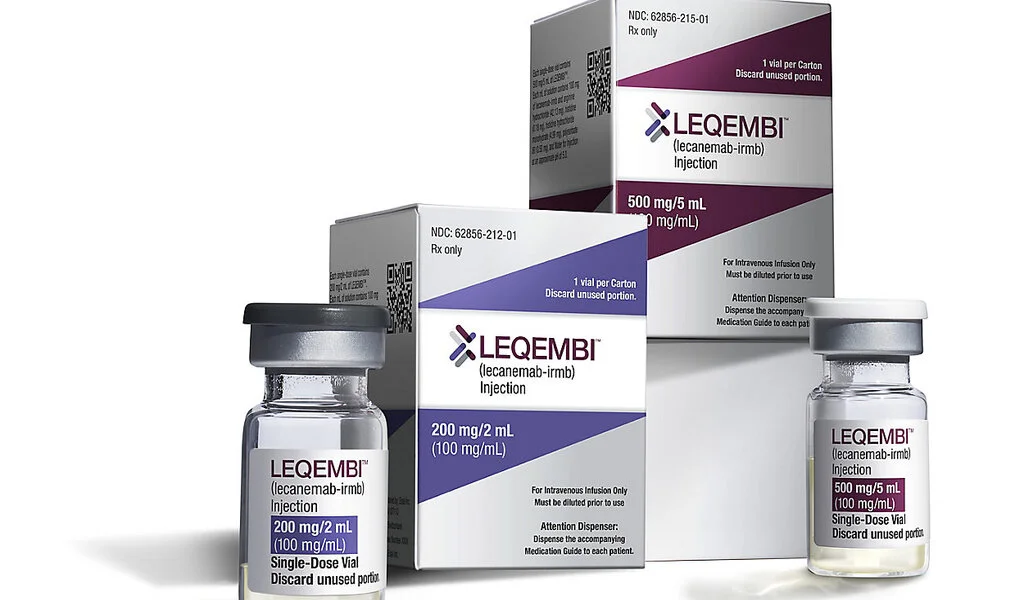
Leqembi represents a beacon of hope for those in the early stages of Alzheimer’s disease. This medication is specifically designed to cater to patients with mild dementia and early-stage symptoms, offering a modest yet significant means to slow down cognitive decline.
Prime Minister Fumio Kishida, who announced this milestone, aptly referred to it as a “breakthrough.”
With the approval of Leqembi, the treatment of dementia in Japan has indeed entered a new era.
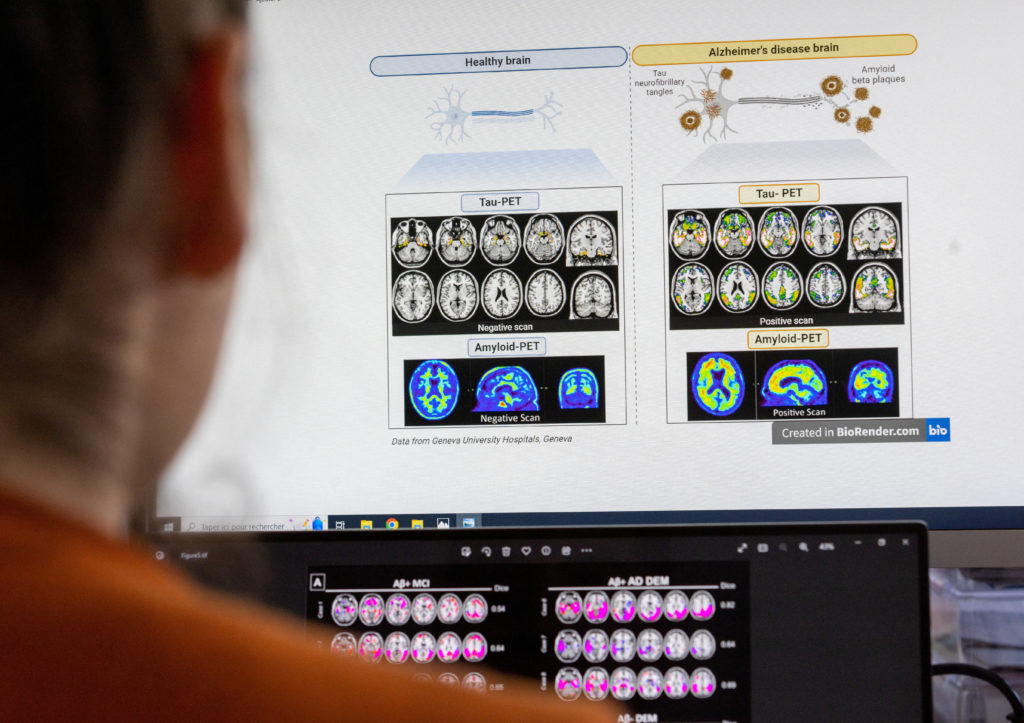
A scientist looks at scans of grains at the Memory Centre at the Department of Readaptation and Geriatrics of the University Hospital (HUG) in Geneva, Switzerland, June 6, 2023. REUTERS/Denis Balibouse
Rising Dementia Cases in Japan
One cannot overstate the urgency of addressing Alzheimer’s disease in Japan. The country is facing an unprecedented demographic challenge, with its aging population set to reach new heights.
According to the health ministry, the number of dementia patients aged 65 or older is expected to rise from 6 million to 7 million by 2025.
This alarming increase necessitates immediate action and innovative solutions to provide adequate care and support to those affected.
Challenges and Considerations
While Leqembi’s approval is undoubtedly a cause for celebration, it’s essential to acknowledge the challenges associated with Alzheimer’s treatment. Notably, the drug may not be effective for everyone, a common issue faced by Alzheimer’s medications.
Additionally, like other drugs targeting brain plaques associated with Alzheimer’s, Leqembi can pose risks, including brain swelling and bleeding, albeit in rare cases. These side effects warrant careful monitoring and patient education.
To address these concerns, Eisai has committed to conducting a post-marketing special use survey. This initiative will collect crucial data from patients administered the drug, ensuring a comprehensive understanding of its real-world effectiveness and potential risks.
Such measures are essential for the responsible introduction of a new treatment.
Affordability and Accessibility
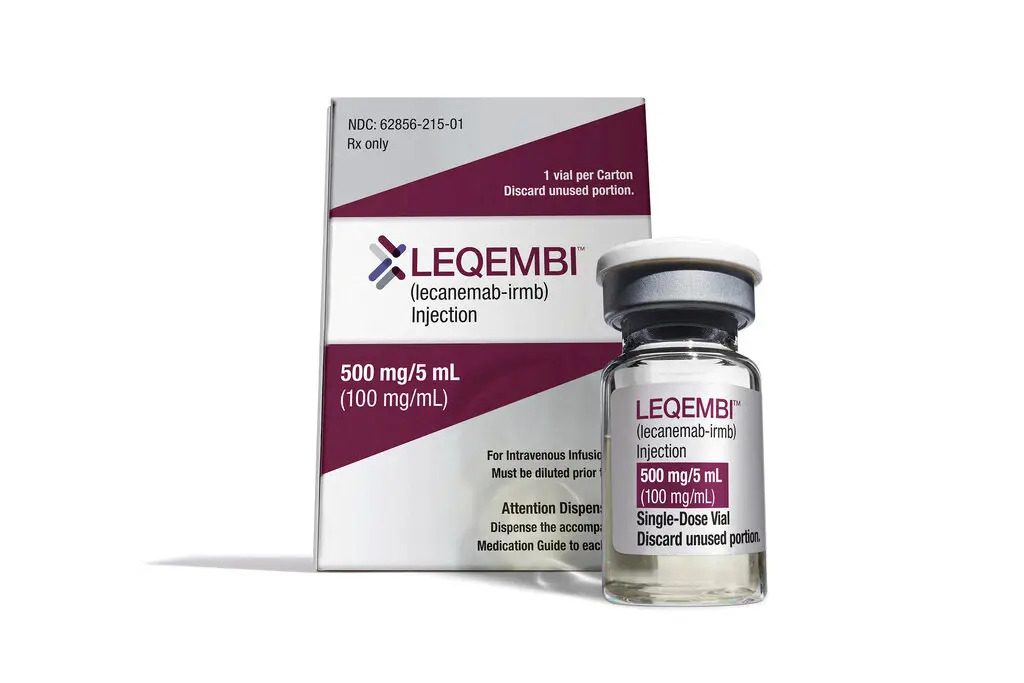
Another crucial aspect to consider is the accessibility of Leqembi. While the drug is expected to be partially covered by health insurance, its price remains undetermined.
Reports from Kyodo News agency suggest that it may be relatively expensive. Ensuring that individuals and families can afford this treatment is paramount, especially given the increasing prevalence of Alzheimer’s in Japan.
Eisai’s Commitment
Eisai Co., the Japanese pharmaceutical company at the forefront of Leqembi’s development, has expressed unwavering dedication to delivering this innovative treatment to those in need.
Haruo Naito, the CEO of Eisai, affirmed the company’s mission to make an impact on the pressing issues surrounding dementia in Japanese society.
This commitment extends beyond the drug’s approval and encompasses ongoing efforts to provide support and solutions for dementia patients and their families.
Pathway to a Dementia-Friendly Society
Prime Minister Kishida’s announcement of Leqembi’s approval coincides with his commitment to bolster support for dementia patients and their families. A forthcoming panel is expected to convene to discuss measures for creating a dementia-friendly society.
This initiative underscores the holistic approach needed to address the multifaceted challenges posed by Alzheimer’s disease.
Conclusion
The approval of Leqembi in Japan marks a momentous stride in the fight against Alzheimer’s disease.
As the country grapples with a rising number of dementia cases and an aging population, this innovative medication offers newfound hope for patients in the early stages of the disease.
While challenges and uncertainties persist, Eisai’s dedication to responsible post-marketing surveillance and affordability underscores the commitment to addressing this pressing health issue.
With Prime Minister Kishida’s vision of a dementia-friendly society, Japan is embarking on a path that acknowledges the urgency of Alzheimer’s care and the importance of comprehensive solutions.
As Leqembi becomes available to patients, it brings with it the promise of a brighter future for those affected by Alzheimer’s disease in Japan.
RELATED CTN NEWS:
U.S. Government Offers Free At-Home COVID-19 Test Kit: How To Order Free Tests?
Thailand Extends Warm Welcome To Chinese Tourists With Visa Waiver Program
Thailand’s FDA Scrutinizes Japanese Imports For Radiation Contamination, Ensuring Seafood Safety