News
FDA Rejects ‘Neffy’ Epinephrine Nasal Spray Despite Positive Advisory Committee Vote
(CTN News) – In an unexpected turn of events, the Food and Drug Administration (FDA) has chosen not to grant approval for an epinephrine nasal spray, which could have marked a groundbreaking, needle-free alternative to epinephrine autoinjectors like EpiPens.
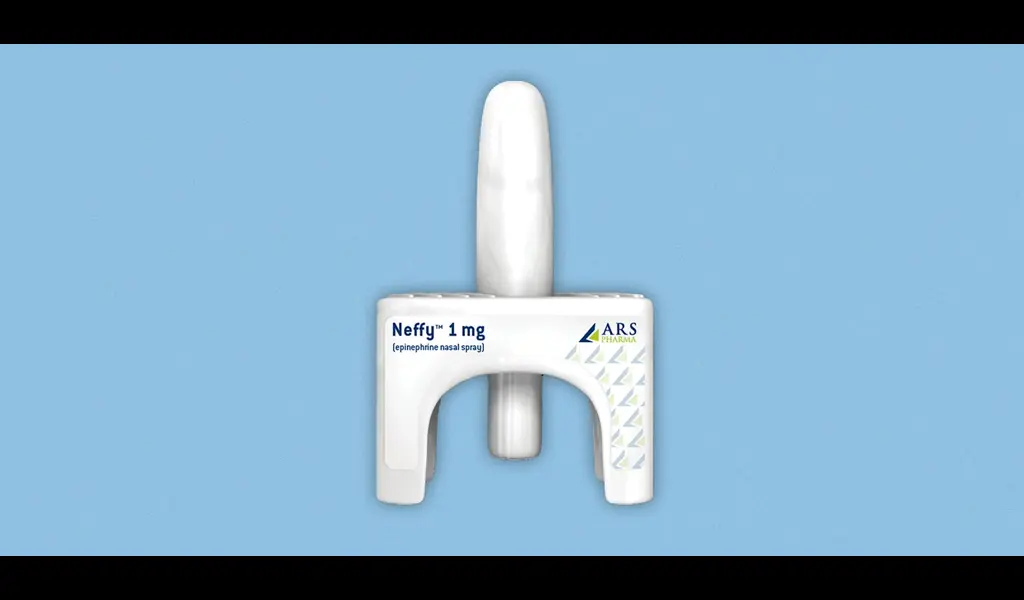
According to a statement released by ARS Pharmaceuticals late Tuesday night, the FDA has requested that the company conduct an additional study on the drug, known as Neffy, in order to provide further evidence to support its approval.
This rejection comes as a surprise, especially considering that the FDA‘s advisory committee had previously voted in favor of recommending Neffy’s approval for use in both children and adults back in May.
It is worth noting that it is relatively rare for the FDA to decline approval for drugs that have received a positive recommendation from its advisory committees.
“We are genuinely taken aback by this development,” expressed Richard Lowenthal, the CEO of ARS Pharmaceuticals, in the company’s official statement.
Nasal Sprays: Types, Uses, and Guidelines
Nasal spray is a medication delivery method used to administer drugs directly into the nasal passages. It is commonly used to treat various conditions, including allergies, congestion, and nasal infections. Here are some key points about nasal sprays:
- Types of Nasal Sprays:
- Decongestant Nasal Sprays: These provide temporary relief from nasal congestion and are available over-the-counter. They work by narrowing blood vessels in the nasal passages.
- Steroid Nasal Sprays: These are used to treat allergies and inflammation. They help reduce inflammation in the nasal passages and can be prescribed by a doctor.
- Saline Nasal Sprays: These contain a saltwater solution and are used to moisturize and flush out nasal passages. They are often used for nasal irrigation.
- Common Uses:
- Allergies: Nasal sprays can alleviate symptoms of seasonal or year-round allergies, such as sneezing, runny nose, and congestion.
- Nasal Congestion: Decongestant nasal sprays can provide quick relief from nasal congestion due to colds or allergies.
- Nasal Polyps: Steroid nasal sprays may be prescribed to reduce inflammation associated with nasal polyps.
- Sinus Infections: They can help manage symptoms of sinusitis, such as sinus pressure and congestion.
- How to Use Nasal Sprays:
- Follow the instructions provided with the specific nasal spray product.
- Typically, you’ll insert the nozzle into your nostril, aim slightly outward, and spray while inhaling gently. Avoid tilting your head back too far.
- After using a nasal spray, it’s important to clean the nozzle to prevent contamination.
- Side Effects:
- Common side effects may include nasal dryness, irritation, or a mild burning sensation.
- Overusing decongestant nasal sprays can lead to a condition called “rebound congestion.”
- Consult a Healthcare Professional:
- If you have chronic nasal symptoms or are considering using a nasal spray long-term, it’s advisable to consult a healthcare provider for proper guidance and to rule out underlying conditions.
Please note that this information is for general understanding, and it’s essential to consult a healthcare professional or read the specific instructions provided with any nasal spray product for proper usage and potential side effects.
ARS Pharmaceuticals to Contest FDA’s Data Demand for Needle-Free Epinephrine
ARS Pharmaceuticals has announced its intention to challenge the FDA’s demand for additional data.
Epinephrine, a life-saving medication that has been in use in the United States since 1901, is highly effective in reversing anaphylaxis, the most severe form of allergic reaction.
Anaphylaxis can occur within minutes of exposure to an allergen, such as peanuts or cat dander, and without prompt treatment, it can prove fatal.
However, all currently available epinephrine treatments require injection, which poses a challenge for individuals with a fear of needles.
Dr. Zachary Rubin, an allergist at Oak Brook Allergists in Illinois, expressed his astonishment at the FDA’s decision, stating, “I’m shocked.” Dr. Rubin, who is not a member of the FDA’s advisory committee, emphasized the lack of viable alternatives at present.
He noted, “Currently, we essentially have epinephrine autoinjector devices, needle-based options, and people have been advocating for years for a needle-free alternative.”
During the advisory committee meeting in May, a major concern revolved around the insufficient clinical data, particularly the absence of studies involving individuals experiencing anaphylaxis.
ARS Pharmaceuticals argued that their nasal spray demonstrated “comparable” effectiveness to an EpiPen, based on research conducted in both animals and individuals who did not have anaphylaxis.
Despite these concerns, the advisory panel ultimately voted 16-6 in favor of the drug for use in adults and 17-5 in favor of its use in children.
Ethical Challenges in Studying Life-Saving Allergy Drug: Dr. Amirshahi’s Perspective
For Dr. Maryann Amirshahi, a professor specializing in emergency medicine at Georgetown University School of Medicine and a member of the advisory committee, the absence of data concerning patients with anaphylaxis remained a significant concern.
Consequently, she cast her vote against the drug on both occasions.
Amirshahi expressed her perspective in an email to NBC News on Tuesday, saying, “I listened to the parents during the hearing, and I heard them discuss the trauma associated with administering a shot to their children.
However, as a parent myself, a panel member, a pharmacologist, and an ER physician who has encountered numerous cases of anaphylaxis, the most concerning aspect for me was not the injection, but the potential failure of a drug to effectively treat a life-threatening condition.”
The ethical challenges of studying a drug designed for emergency treatment of life-threatening allergic reactions are complex.
Conducting a randomized controlled trial, in which both adults and children experiencing severe allergic reactions are given either the treatment or a placebo, would be ethically problematic, according to Rubin.
Amirshahi suggested that it might be feasible to study the drug in individuals experiencing anaphylaxis in controlled settings like an allergist’s office or an emergency room, where there would be a backup treatment readily available.
She expressed hope that the FDA’s rejection would provide an opportunity to conduct comprehensive research on the drug to ensure its effectiveness. She remarked, “While I believe the drug has potential, it currently lacks sufficient efficacy data.”
In response to the FDA’s decision, the agency has requested an additional study comparing Neffy to an epinephrine autoinjector in individuals experiencing nasal symptoms induced by allergens, such as sneezing, itching, and congestion.
The drug manufacturer has indicated its intention to resubmit the application to the FDA in the first half of 2024.