Health
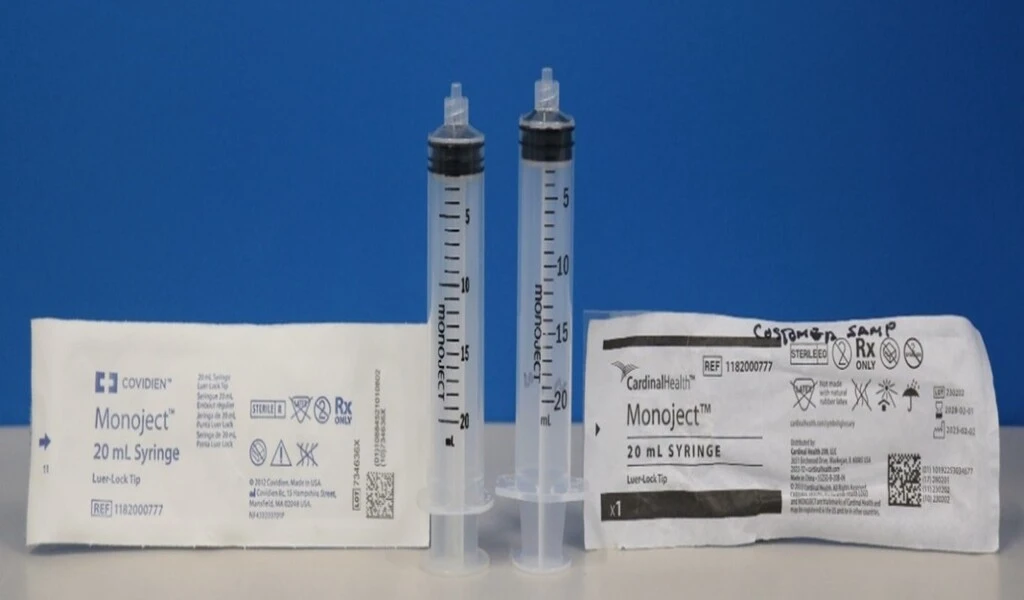
FDA Warns Against Cardinal Health’s Monoject Syringes in Pain Management
(CTN News) – The recent FDA warning against using Cardinal’s syringes has sent shockwaves through the medical community. This article delves into the details of the warning, the issues identified, and the broader implications for both consumers and the medical equipment industry.
Cardinal’s Syringes: What went wrong?
Cardinal’s syringes, once deemed reliable, are now under scrutiny due to identified issues that prompted the FDA warning. These issues range from design flaws to manufacturing defects, raising concerns about the safety and efficacy of these medical devices.
Health Risks Associated
The FDA warning highlights potential health risks associated with using Cardinal’s syringes. Users may face complications ranging from incorrect dosages to contamination, posing serious threats to patient well-being. It’s crucial for healthcare providers and consumers to be aware of these risks.
FDA’s Regulatory Measures
In response to the identified issues, the FDA has implemented regulatory measures to mitigate the risks associated with Cardinal’s syringes. This section explores the steps taken by the FDA to address the situation and ensure the safety of medical equipment users.
Alternative Safe Options
Amid the FDA warning, it’s essential to identify alternative syringe options that meet safety standards. Healthcare professionals are advised to explore and adopt alternative brands that prioritize patient safety.
Importance of FDA Warnings
The FDA plays a pivotal role in safeguarding public health. Understanding and heeding warnings issued by the FDA is paramount to preventing potential harm to patients. This section emphasizes the importance of taking such warnings seriously.
Cardinal’s Response
How has Cardinal responded to the FDA warning? This section provides an overview of Cardinal’s actions, addressing the issues raised and outlining their commitment to rectifying the situation.
User Feedback and Concerns
Public reaction to the FDA warning has been significant. Users and healthcare providers alike have expressed concerns about the safety of medical devices. This section explores the feedback received and the concerns raised by those directly affected.
Industry Impact
The FDA warning doesn’t just affect Cardinal but has broader implications for the medical equipment industry. This section examines how such warnings impact industry practices, regulations, and consumer trust.
Ensuring Patient Safety
Healthcare professionals play a crucial role in ensuring patient safety. This section discusses the responsibilities of medical practitioners in choosing reliable and safe medical equipment for their patients.
Legal Implications
Beyond the immediate safety concerns, there may be legal consequences for Cardinal. This section briefly touches upon potential legal implications and actions that affected parties may take.
Public Awareness Campaigns
Raising awareness about the FDA warning is essential for public safety. This section explores the importance of public awareness campaigns to inform consumers about potential risks and how to navigate them.
Learning from Past Incidents
The FDA warning against Cardinal’s syringes is not the first of its kind. Reflecting on past incidents involving medical devices helps us understand the need for continuous improvement in safety standards.
What Consumers Can Do
Empowering consumers with knowledge is crucial. This section provides practical advice to consumers on ensuring the safety of the medical devices they use, emphasizing vigilance and informed decision-making.
Conclusion
In conclusion, the FDA warning against using Cardinal’s syringes underscores the importance of rigorous safety standards in the medical equipment industry. It serves as a reminder for both manufacturers and consumers to prioritize patient safety above all else.