(CTN NEWS) – Despite a long list of concerns about how to effectively guard against a virus that is still quickly changing, the United States is ready to make COVID-19 immunisations more similar to an annual flu shot, a significant shift in policy.
The Food and Drug Administration asked its scientific advisors on Thursday to help create the basis for most Americans to transition to once-yearly boosters and to determine how often the shot’s formula should be updated.
According to Dr. David Kaslow of the FDA, “This is a significant conference to assess if we’ve reached the point in the pandemic that permits for streamlining the use of current COVID-19 vaccinations.”
The FDA’s strategy was largely endorsed by the advisory council.
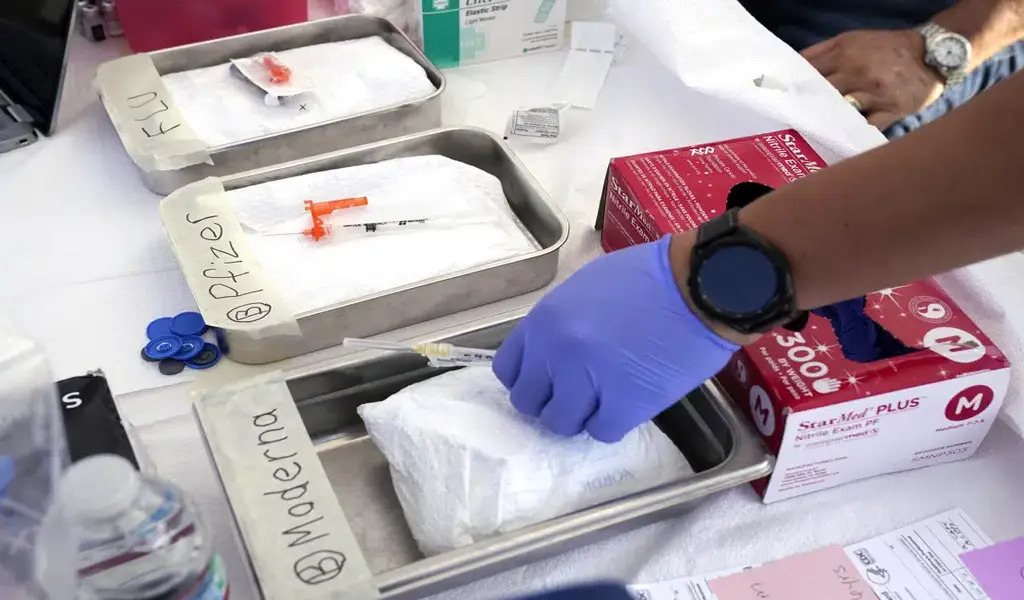
Millions of lives have been saved by 19 vaccinations, and even if more contagious varieties have emerged, booster doses continue to benefit the most vulnerable.
The shots don’t last very long, and protection does eventually fade against more minor illnesses.
And vaccinations are becoming boring for people.
Over 80% of Americans have received at least one COVID-19 vaccination, but just 16% of individuals who are eligible for the most current boosters—so-called bivalent doses revised to better match more recent virus strains—have received one.
This necessitates difficult choices about how to proceed. Who, how frequently, and for what actually needs another shot?
Even when the most recent altered omicron strains surfaced, FDA adviser Dr. Paul Offit, a vaccine specialist at Children’s Hospital of Philadelphia, said, “We’re still protected against serious sickness, thank god.”
The FDA advisory panel unanimously decided that regardless of whether someone is receiving their first immunisation or a booster shot, they should receive the same vaccine formulation.
For their initial two or three doses, Americans now receive a single formulation based on the original coronavirus strain that first appeared in 2020.
Their most recent booster is a combo shot produced by Pfizer or Moderna that includes omicron protection. The FDA would have to choose how to introduce that modification gradually.
To clear up confusion about such vaccinations, however, “this isn’t merely a convenience item,” claimed Dr. Archana Chatterjee, head of Chicago Medical School. Moving toward the circulating coronavirus strains is crucial because the original coronavirus strain has vanished.
When more discussion arose and who needed another chance.
The FDA predicted that most Americans should be fine if they receive a once-yearly booster in the fall that is specifically tailored at the newest versions.
The organisation questioned if some individuals—adults with compromised immune systems and very young infants who have never received a vaccination before—might require two doses. That’s comparable to how children receive their first flu shot.
More information, such as a meticulous count of people who still get hospitalized with COVID-19 while being current on their immunisations, is required to show precisely who could need two yearly doses, according to Offit.
Only then, he continued, “can we really make the greatest choice as to who gets immunized with what and when.”
It’s also unclear whether COVID-19 boosters are required annually for younger, healthier individuals. Dr. Eric Rubin of Harvard University said, “At this moment, it’s difficult to determine if it will be yearly.”
According to FDA advisor Dr. Arthur Reingold of the University of California, Berkeley, fall may not even be the optimal time to boost, depending on when infections increase and how long a booster’s protection might continue.
Contrary to flu, which primarily circulates in the late fall and winter in the United States, COVID-19 waves have occurred all year long.
The FDA intends to convene its advisory council for a new meeting in late May or early June to determine whether the vaccine recipe needs to be modified.
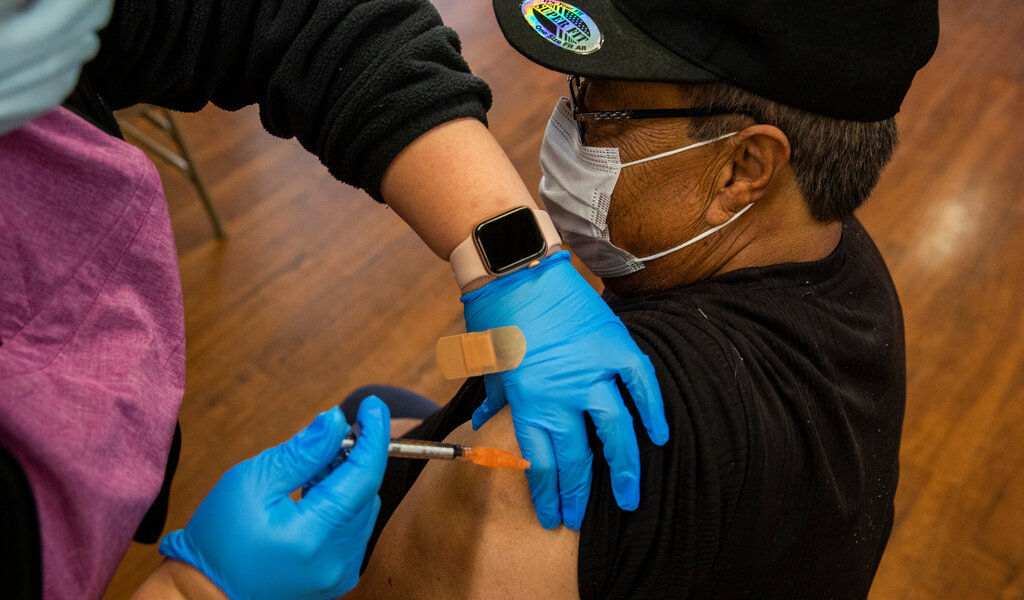
This will include determining which virus strain to target and whether a single-strain or multi-strain shot is preferable.
While a third producer, Novavax, advocated an earlier start to any recipe change, Pfizer and Moderna claimed it would give enough time to generate the needed doses by fall.
Also on Thursday, U.S. officials provided an update on how they are monitoring the safety of the most recent COVID-19 boosters.
The Centers for Disease Control and Prevention have identified a potential stroke warning sign that could apply to seniors receiving Pfizer’s revised booster.
The government concluded that it’s doubtful the red flag was genuine when FDA safety specialist Richard Forshee pointed out that data from Medicare and numerous other health systems, including those in other nations, found no indication of trouble.
RELATED CTN NEWS:



