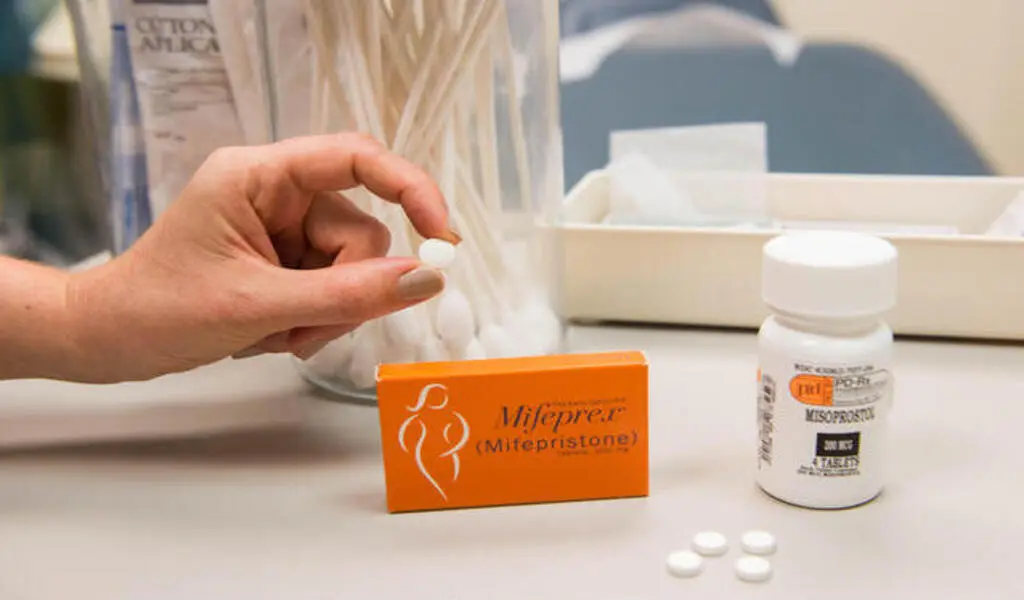
(CTN News) – Since the U.S. Supreme Court overturned its historic 1973 decision in Roe v. Wade, which had protected abortion rights globally, last June, medication abortion has been under the spotlight.
Two lawsuits aim to increase access to the medicine used in the surgery, while one seeks to remove it from the market. What is at risk is explained below.
WHAT IS MEDICATION ABORTION?
Medication abortion is a two-drug regimen consisting of mifepristone followed by misoprostol used to end a pregnancy during the first 10 weeks and accounts for more than half of all abortions performed in the United States.
WHAT ARE THE LAWSUITS?
In a federal court in Amarillo, Texas, anti-abortion organizations filed a lawsuit against the U.S. Food and Drug Administration in November, alleging that the government approved the abortion drug mifepristone in 2000 without giving it the consideration it deserved.
While the action is pending, the organizations request a preliminary order, sometimes known as an injunction, to invalidate the FDA’s clearance.
Last month, two different cases were brought to increase access to mifepristone.
In one, the manufacturer of generic mifepristone GenBioPro is requesting that a federal court prevent West Virginia, which has an almost complete abortion ban, from outlawing the sale of the tablets.
On the other, a physician from North Carolina is attempting to overturn the state’s drug regulations, which include the need for patients to physically get the medication from a doctor at specifically regulated facilities after undergoing required counseling.
The state is one of 16 which permits certain forms of abortion but has extra limitations on mifepristone, making it more difficult to get.
Both depend on a well-known legal principle called federal preemption, which states that federal law — in this instance, the FDA’s power to approve and regulate medications — prevails over incongruous state legislation.
WHAT WOULD HAPPEN IF THE TEXAS PLAINTIFFS WIN?
Since federal courts sometimes have the authority to impose injunctions that go beyond the boundaries of their districts to include the whole nation, a plaintiffs’ success in Texas might result in the full eradication of mifepristone from the market nationally.
In a recent court filing, the FDA said that taking the medication off the market would significantly injure patients by making them undergo needless and sometimes dangerous surgical abortions and exposing them to long wait periods.
The prospect of prescribing misoprostol alone for medication abortion, which is not an FDA-approved usage but is in certain other nations, has been brought up by abortion providers.
Although such off-label prescription is typically permitted, it is unclear how many healthcare professionals would use it.
IS A WIN BY TEXAS PLAINTIFFS LIKELY?
Legal experts claim there is no precedence for challenging FDA clearance after the fact based on purported safety concerns, and plaintiffs will need to provide a compelling legal argument to get beyond the standard six-year statute of limitations.
However, by choosing to file in Amarillo, the plaintiffs have made sure that their case will be heard by U.S. District Judge Matthew Kacsmaryk, a vocal conservative nominated to the federal court by former Republican President Donald Trump who has previously supported conservative causes.
WHAT WOULD HAPPEN NEXT?
While it files an appeal with the 5th U.S. Circuit Court of Appeals, the FDA will ask for an urgent injunction stay. Given that most of the justices on that court were chosen by Republicans, it is also regarded as a conservative court.
However, some proponents of abortion rights have said that they think it would be prudent to withdraw an FDA-approved medication.
Regardless of the 5th Circuit’s decision, the case will probably be appealed to the US Supreme Court.
WHEN COULD THERE BE A RULING?
It’s unclear when Kacsmaryk will rule. Both parties have until Friday to file documents indicating whether the court should convene a hearing on a preliminary injunction or go straight to a full trial on the merits.
He has given the plaintiffs until February 24 to reply to Danco Laboratories LLC, the firm that makes the brand name mifepristone, in its application contesting the injunction.
After receiving these submissions, Kacsmaryk might set a hearing or trial date.
WHERE DO THE ACTIONS IN WEST VIRGINIA AND NORTH CAROLINA STAND?
Because both cases explore unexplored legal territory, it isn’t easy to foresee how they will turn out. The North Carolina case, though, is more likely to succeed.
When Massachusetts attempted to outlaw an FDA-approved opioid painkiller, federal preemption for prescription pharmaceuticals was put to the test in court.
A court invalidated such restriction due to preemption issues. According to legal experts, the same justification would seem to exclude states from adopting further safety regulations on mifepristone beyond those established by the FDA.
Since West Virginia’s abortion prohibition covers all abortions and does not expressly address mifepristone, the West Virginia complaint advances a more creative legal defense.
GenBioPro contends that because it effectively stops the drug’s sales, it should be viewed as a prohibition on mifepristone and is thus preempted.
However, the state will probably argue that regardless of how abortion is carried out, it has the authority to control it.
COULD THERE BE MORE LEGAL ACTION?
Many experts predict that there will be. A group of Republican attorneys general cautioned large pharmacies on February 1 that distributing mifepristone tablets by mail might violate state and federal law, which may be a portent of things to come.
A week later, Texas filed a lawsuit against the Biden administration for telling pharmacists they couldn’t refuse to fill prescriptions for contraceptives.
Related CTN News:
China has 80% of Covid-19 infected People, So There’s Little Chance of a Big Rebound