(CTN News) – The US Food and Drug Administration (FDA) has issued a high-level notice on a cardiac pump that has been related to 49 deaths and 129 injuries.
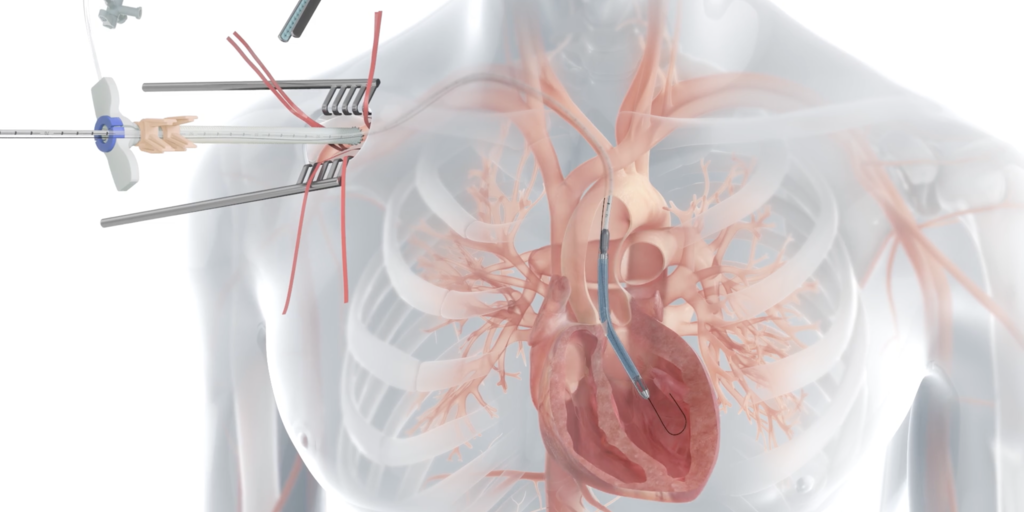
The Impella left-sided pumps provide temporary heart assistance to patients undergoing high-risk surgeries or recovering from a major heart attack.
However, the regulator warned that if used incorrectly, it might pierce a wall in the heart’s left ventricle.
Abiomed, the device’s manufacturer, has updated the pump’s instructions.
According to a statement on the FDA’s website on March 21, the move is the “most serious type of recall” since the equipment poses a risk of serious injury or death if used incorrectly.
The FDA cautioned that using damaged pumps could result in serious health implications such as “hypertension, lack of blood flow, and death”.
However, it clarified that the recall was a remedy, not a product removal and that the gadget would remain on the market.
According to the government, the notification covers 66,390 devices supplied in the United States over two years beginning on October 10, 2021.
The device received FDA approval in 2008.
The pump has a catheter with a small hook at the end that is threaded through the blood vessels and into the left ventricle, a critical chamber in the heart that pumps oxygenated blood throughout the body.
A representative for Johnson & Johnson, which acquired Abiomed in 2022, told Reuters that “this notification is not a device removal, and Impella heart pumps remain on the market and available for patients.”
According to the regulator, Abiomed originally revealed the risk of cardiac perforation during pump implantation in a technical bulletin published in October 2021 but did not notify the FDA at the time.
The government investigated the firm’s Massachusetts location in early 2023 and sent a warning letter to Abiomed in September, criticizing, among other things, its failure to notify the FDA of the danger of heart perforation.
The warning letter prompted Abiomed to issue an “Urgent Medical Device Correction letter” late last year, according to the FDA. This letter included revised instructions for properly utilizing the heart pump, such as how to position the pump’s catheter or employ imaging when turning it during procedures.