(CTN News) – In March, the Supreme Court will hear a case involving mifepristone, one of two medicines used in medication abortion.
A significant question in that case is whether the Food and Medicine Administration was accurate when it determined that the medicine was safe to prescribe to patients via virtual appointment.
Research published Thursday in Nature Medicine supports the FDA’s opinion that pharmacological abortion is safe and effective.
Researchers reviewed the electronic medical data of almost 6,000 patients from three abortion providers using telemedicine. They also performed an opt-in survey with 1,600 patients.
Some abortion patients communicated with a provider via video, while others utilised a secure chat network comparable to texting.
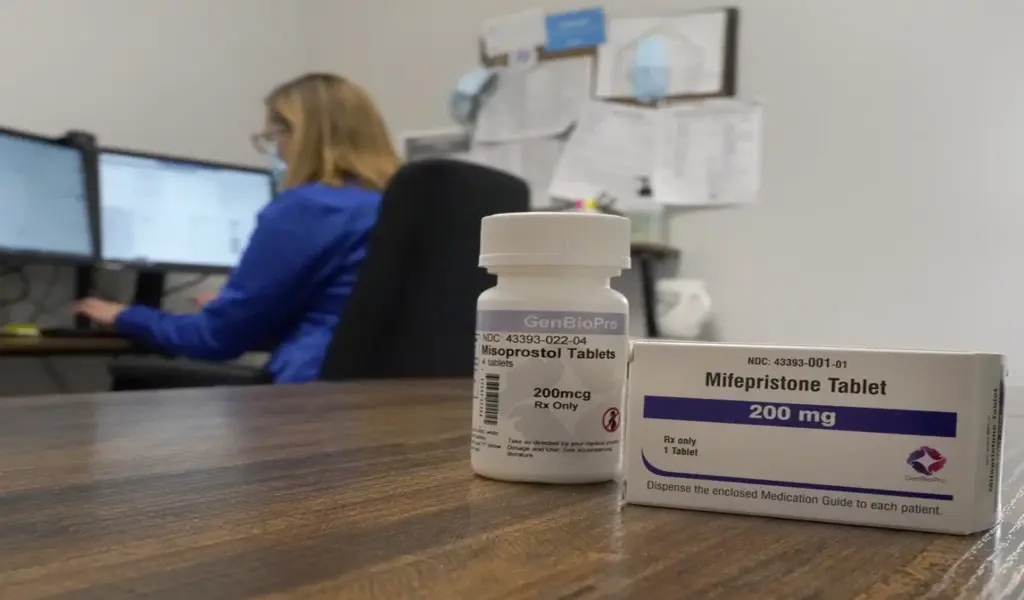
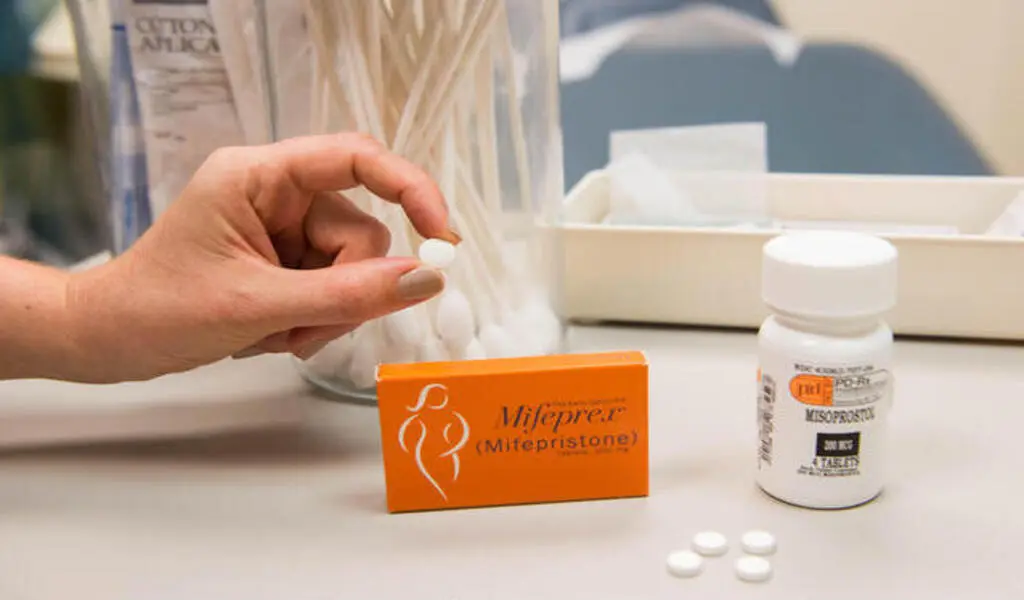
If patients were fewer than 10 weeks pregnant and otherwise eligible, the clinicians recommended two medications: mifepristone, which inhibits the pregnancy hormone progesterone, and misoprostol, which produces uterine contractions. Patients received both medications via mail-order pharmacy.
The study’s lead author, Ushma Upadhyay from the University of California – San Francisco, notes that a clinical follow-up was conducted 3-7 days later. “The provider checked in with the patient.”
‘Have you received your medications? Did you take your medications?’ They inquired about symptoms. Then, there was a clinical follow-up four weeks after the initial intake.
The researchers discovered that the medicine was effective, as it stopped pregnancy in 97.7% of patients without requiring more follow-up therapy.
It was also discovered to be safe; no major adverse outcomes followed 99.7% of abortions. The safety and efficacy were identical whether patients communicated via video or secure chat with a clinician.
“These results shouldn’t be surprising,” Upadhyay says. “It’s consistent with the over 100 studies on mifepristone that have affirmed the safety and effectiveness of this medication.”
The findings are also consistent with worldwide research on telehealth abortion and studies of pharmaceutical abortion administered in a clinic during an in-person session, she adds.
Rishi Desai, a pharmaceutical safety expert from Harvard Medical School, was not involved in the study. He adds that the study was “well-conducted,” especially given the difficulty of tracking patients who only communicate with clinicians remotely.
“I would say that this study provides reassuring data regarding safety of the medications, and this is very much in line with what we have seen in many previous studies,” he said. “So it’s good to see that safety findings hold up in this setting as well.”
Overview of the legal challenges surrounding mifepristone, including the 2022 lawsuit against the FDA and the upcoming Supreme Court hearing on March 26.
Still, whether mifepristone is safe and whether the FDA has adequately regulated how it is supplied is a legal issue.
An anti-abortion rights group sued the FDA in 2022, claiming that mifepristone is unsafe and was unlawfully approved in 2000. Judge Matthew Kacsmaryk, a district court judge appointed to the federal bench by President Trump, decided that mifepristone should be taken off the market worldwide.
Although his ruling did not go into effect while appeals were underway, the appeals court ruled against the FDA in part, notably limiting telemedicine abortion access. That is also currently on hold.
The Supreme Court will hear arguments in the matter on March 26. The ruling might have an impact on access to medication abortion across the country and establish a new precedent for challenges to the FDA’s jurisdiction.
Recently, there has been a surge in mifepristone research headlines. A report citing safety issues concerning mifepristone was recently retracted. This study, published on Thursday, supports the FDA‘s position that the drug can be safely prescribed remotely.
Upadhyay claims she has been working on this research for years and that the timing of its publication, weeks before the Supreme Court arguments, is coincidental.
“I don’t know if they can enter new evidence into the case at this point,” she said. “But I do hope it impacts the perception of how safe this medication is.”