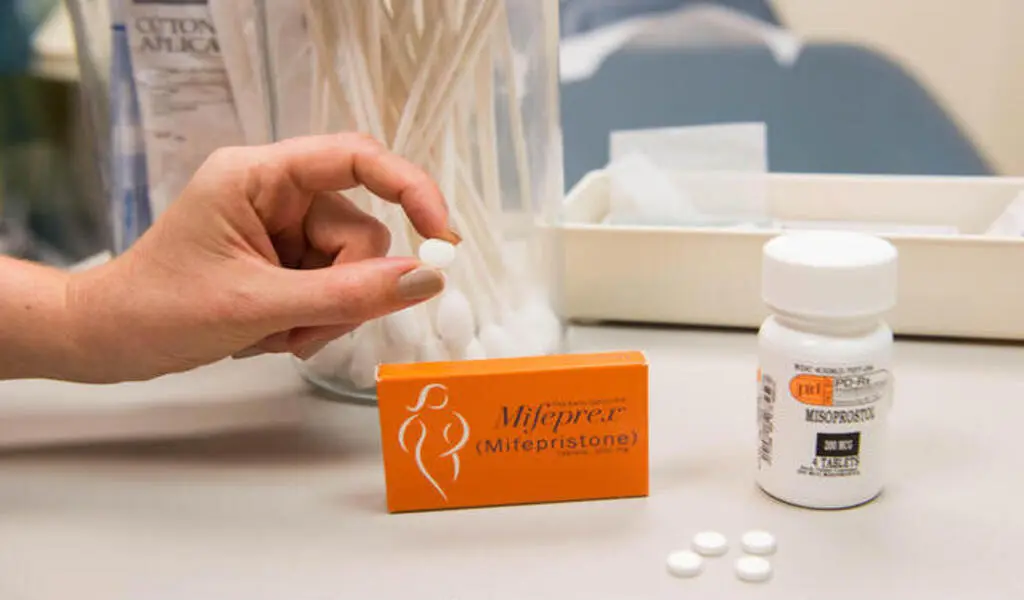
(CTN News) – On Thursday, a federal district judge in Washington state ruled that a ruling by a federal appeals court that limited access to the abortion drug mifepristone would not take effect.
Seventeen states and the District of Columbia sued to protect access to mifepristone, and on Friday, Judge Thomas Rice of the U.S. Eastern District of Texas ordered the Food and Drug Administration to do so.
U.S. Supreme Court to Decide on Future of Mifepristone
In a court ruling issued on Thursday, Rice reaffirmed that the FDA has no authority to restrict patients’ access to the drug, despite a judgment by the U.S. 5th Circuit Court of Appeals this week that limited the medication’s distribution and use.
On Thursday morning, Washington state’s attorney general, Bob Ferguson, told CNBC that a federal judge’s ruling in his state would not be overturned by a court in Texas or the 5th Circuit. Ferguson leads the lawsuit to protect access in the 17 states and the District of Columbia.
Dueling court decisions on the legal status of the medicine have created a confusing legal picture, as highlighted by Rice’s order on Thursday. The United States Supreme Court may soon decide the fate of mifepristone.
Arizona, Colorado, Connecticut, Delaware, Illinois, Michigan, Nevada, New Mexico, Oregon, Rhode Island, Vermont, New Hampshire, Hawaii, Maine, Maryland, Minnesota, Pennsylvania, Washington, and the District of Columbia are all included in Rice’s mandate.
Rice’s directive maintains the status quo of the FDA’s existing regulatory structure in those areas. Mifepristone may be administered up until the 10th week of pregnancy, and abortion pills may be mailed to patients or dispensed at retail pharmacies by trained staff.
We have an extremely clear judgment, and we fully believe that the FDA will honor it,” Ferguson said earlier on Thursday.
Just 20 minutes earlier on Friday, U.S. District Judge Matthew Kacsmaryk of the U.S. Northern District of Texas had suspended the FDA’s approval of mifepristone, which had been in place for over two decades.
On Monday, the Department of Justice appealed with the 5th Circuit Court of Appeals against Kacsmaryk’s ruling.
On Wednesday, a three-judge panel voted 2-1 to reject Kacsmaryk’s request to temporarily halt the Food and Drug Administration’s clearance of mifepristone. However, it did so while restricting access to medicine via stricter regulations.
For now, Trump appointee judges Kurt Engelhardt and Andrew Oldham have voted to reinstate in-person doctor visits as a prerequisite to obtaining the abortion pill.
Due to the judgment, Mifepristone can now only be given up until the seventh week of pregnancy. Oral arguments will be scheduled as soon as possible by the 5th Circuit.
The DOJ requested clarification from Rice on the government’s legal obligations under his order by this coming Friday after identifying a “significant” disagreement between his decision and that of Kacsmaryk.
On Thursday, Rice stated that the decision by Kacsmaryk and the 5th Circuit Court of Appeals ruling do not affect the availability of mifepristone in the 17 states and the District of Columbia.
An earlier statement by Ferguson on Thursday indicated that he did not believe that the FDA would determine for Washington and the states that joined our alliance to pull back access in the manner envisioned by the 5th Circuit.
Biden Administration Appeals 5th Circuit’s Decision to Supreme Court
After maintaining his order safeguarding access, Judge Rice responded to the government’s request for clarification by saying the FDA now has a stronger argument for the court to intervene. This was according to former DOJ attorney Glenn Cohen.
According to Cohen, a health law expert at Harvard Law School, “the need to go to the Supreme Court in the interim becomes more compelling — and FDA has a stronger argument for Court review since two courts are telling it to do opposite things.” This was Cohen’s email to CNBC before Rice’s clarification on Thursday.