Health
U.S. Approves Alzheimer’s Drug That Slows The Disease Significantly
(CTN NEWS) – WASHINGTON – U.S. health officials on Friday approved an Alzheimer’s medicine that moderately delays the brain-robbing disease. Patients and their doctors must carefully assess the drug’s safety concerns.
Leqembi is the first medicine to delay Alzheimer’s memory loss by attacking biochemistry. FDA authorised it for early-stage Alzheimer’s patients.
Leqembi is a remarkable triumph in a field accustomed to failed experimental treatments for the incurable illness. The medicine may only delay cognitive deterioration by a few months, but experts say it could enhance life.
It’s not a cure. Dr. Joy Snider, a neurology at Washington University in St. Louis, says it slows the disease’s course. That might add six to 12 months to someone’s driving privileges.
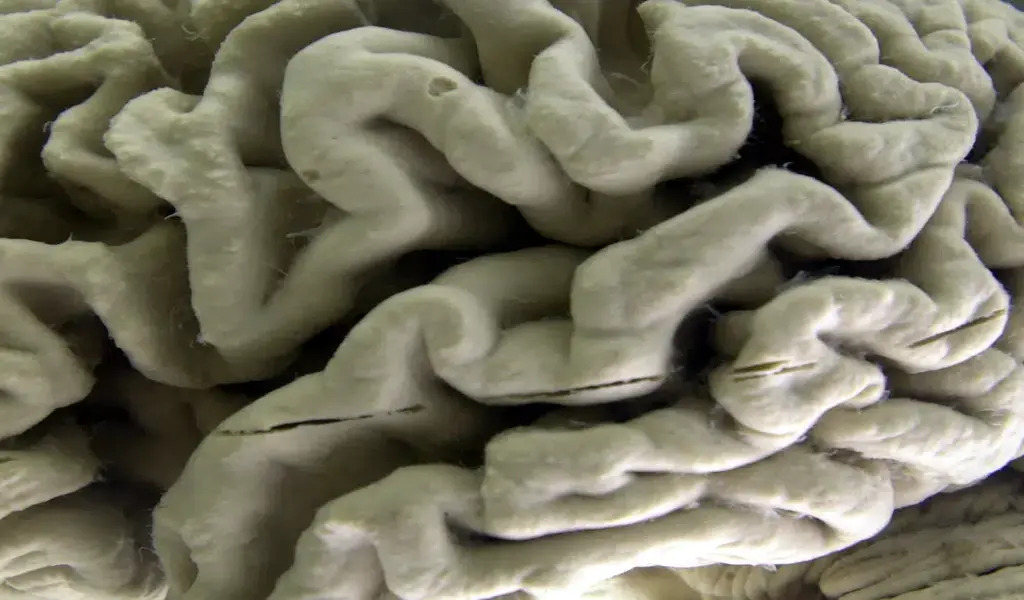
This Oct. 7, 2003 file photo shows a closeup of a human brain affected by Alzheimer’s disease, on display at the Museum of Neuroanatomy at the University at Buffalo in Buffalo, N.Y. On Friday, Jan. 6, 2023, U.S. (AP Photo/David Duprey)
Snider noted that the drug requires twice-monthly injections and may cause brain swelling.
The FDA’s fast pathway allows medications to debut based on early data before they’re proven to benefit patients. Government watchdogs and congressional investigators are scrutinizing the agency’s shortcuts.
A congressional report concluded that the FDA’s approval of Biogen and Eisai’s Alzheimer’s medicine Aduhelm was “rife with irregularities,” including unauthorized meetings with pharma industry workers.
As insurance determines whether to fund lecanemab, most patients won’t receive it for months.
A year’s treatment costs $26,500. Eisai claimed the pricing reflects the drug’s better quality of life, reduced carer obligations, etc. The business said it priced it lower to save patients and insurance money.
An impartial drug-valuation committee claimed the medicine must cost less than $20,600 per year to be cost-effective.
Alzheimer’s attacks memory, thinking, communication, and daily duties in 6 million Americans and many more globally.
Biogen estimated there would be little correlation between price and demand — unless "public scrutiny" became a problem. pic.twitter.com/Ni33nl2pcC
— Damian Garde (@damiangarde) December 29, 2022
The FDA approved the drug based on a mid-stage study of 800 persons with early Alzheimer’s who could live independently or with minimal help.
Since then, Eisai has released a 1,800-patient trial that the FDA will analyze to validate the drug’s benefit, paving the road for final clearance later this year.
The bigger study measured memory, judgment, and other cognitive functions on an 18-point scale. Doctors interview the patient and a close friend.
After 18 months, Leqembi patients dropped less than half a point slower than dummy infusion patients. Five months passed.
There’s limited agreement on whether this difference gives patients greater independence.
Neurology expert Dr. Matthew Schrag says most patients won’t notice the difference. This effect is minor and presumably not clinically meaningful.
Schrag and other experts feel a substantial improvement requires at least one point on the 18-point scale.
Leqembi clears the Alzheimer’s-related protein amyloid. The disease’s cause is unknown. Many amyloid-targeting medicines have failed, therefore researchers increasingly recommend combo therapies.
Aduhelm’s usefulness was disputed.

This Dec. 21, 2022 image provided by Eisai in January 2023, shows vials and packaging for their medication Leqembi. On Friday, Jan. 6, 2023, U.S. health officials approved Leqembi, a new Alzheimer’s drug that modestly slows the brain-robbing disease.(Eisai via AP)
The FDA authorized that medicine in 2021 against outside experts’ advice. Doctors hesitate to prescribe, and insurance limits coverage.
The same panel didn’t approve Leqembi.
Schrag said many of the same issues apply to the new medicine despite “reduced drama.”
“Is this modest, demonstrable gain worth the price and adverse effects?” he wondered. “I’m skeptical.”
13% of Eisai’s research participants had brain edoema and 17% had minor brain bleeds, negative effects of earlier amyloid-targeting drugs. These issues rarely create symptoms like dizziness and vision impairment.
Several Leqembi users died, including two on blood thinners. Eisai says the deaths aren’t drug-related. The FDA warns against prescribing Leqembi to blood-thinner patients.
Insurers will likely only cover the medicine for those with modest symptoms and confirmed amyloid accumulation, like in the corporate research. Brain scans are usually needed. Monitor brain swelling and haemorrhage with a separate scan.
Today, we approved a new medication for the treatment of Alzheimer’s disease, the second of a new category of medications approved for Alzheimer's that target the fundamental pathophysiology of the disease. https://t.co/ZXJCUuTYPk pic.twitter.com/TV36k6CO6K
— U.S. FDA (@US_FDA) January 6, 2023
Medicare covers 60 million seniors and other Americans and will decide whether to cover the medicine. The FDA significantly curtailed Aduhelm coverage, wiping down its U.S. market and forcing Biogen to halt marketing efforts.
Eisai executives have spent months talking to Medicare regulators about their drug. After the FDA certifies the drug’s benefit, coverage is expected later this year.
Once Medicare decides, Eisai’s U.S. CEO Ivan Cheung can launch the medicine nationwide.
Betsy Groves, 73, was diagnosed with Alzheimer’s in 2021. She had problems recalling student names and responding to questions as a Harvard education lecturer.
A positive amyloid test validated her cognitive exam-based diagnosis. Groves is “willing” to try Leqembi despite side effects and infusions.
“I’ll take it as soon as my doctor approves it,” Groves added.
RELATED CTN NEWS:
COVID-19 Risk To Be Minimize During Travel Rush In China