Covid-19
FDA Approves Bivalent COVID Shots for Kids as Young as 6 Months Old

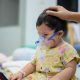
(CTN News) – The COVID Shots from Moderna (MRNA.O), Pfizer (PFE.N), and its partner BioNTech (22UAy.DE) are approved for use in infants as young as six months old. They target both the original coronavirus and Omicron sub-variants.
The Food and Drug Administration approved the use of Moderna’s bivalent injection as a booster dose in children aged 6 months to 5 years on Thursday, two months after the first immunization.
For individuals aged 6 months to 4 years who have not finished their main immunization series or have not yet received the third dosage, Pfizer/revised BioNTech’s vaccine may now be administered as a third dose.
According to the organization, children who have finished their first three doses of the Pfizer original vaccine are not yet eligible for the bivalent booster.
The agency also said that in January, data in favour of using the bivalent injection from Pfizer/BioNTech as a booster in this age range is anticipated.

The earliest children in the United States were the final group to become eligible for immunization since COVID Shots weren’t officially authorized until June of this year.
As of November 30, just 2.7% of eligible children under the age of two and fewer than 5% of those between the ages of two and four had finished their main immunization series, according to government statistics.
This represents a delayed absorption of the first vaccine doses in young children. Children under six may get the two-dose, 25-microgram Moderna vaccine, spaced around four weeks apart.
The Pfizer/BioNTech vaccine is a lower dosage, three-shot regimen administered over a minimum of 11 weeks. The Centers for Disease Control and Prevention said that as of Nov. 30, 39.7 million Americans had gotten a bivalent booster.
When combined, the BQ.1 and BQ.1.1 subvariants currently account for most infections in the U.S. and have replaced Omicron BA.5.

Because they are more immunologically evasive than BA.5, the new COVID Shots probably won’t function as well against the BQ subvariants, but they are still anticipated to provide significant protection against serious diseases.
The FDA approved the doses based on adult immune response data for comparable vaccines created by Pfizer and Moderna that target the original omicron BA.1 mutation.
The safety data is also based on data from the BA.1 clinical trial and other studies that assessed the effectiveness of the primary vaccination as a booster.
Related CTN News:
U.K. Approves New Moderna Vaccine Targeting Omicron Variant